Accuracy of forearm dimensions obtained with a three-dimensional surface scanner
Timon Peter ter Braak1, Huu Chien Nguyen1, Lysanne van Silfhout2*, Albert Frederik Pull ter Gunne2, Edo Johan Hekma2
1University of Twente, Enschede, The Netherlands
2Department of Trauma Surgery at Rijnstate Hospital, Arnhem, The Netherlands
Abstract
Background: Three-dimensional (3D) printed orthoses are being investigated as potential replacements for conventional casts for fracture treatment. 3D-printed casts could improve patient comfort and outcomes, as well as reduce complications like neuropraxia or cutaneous disease. Dimensions necessary to create such casts could be obtained in an easy, quick and non-invasive way with 3D surface scanner. The objective was to validate the Structure Sensor 3D scanner for forearm measurements.
Methods: The Structure Sensor 3D scanner was used to take scans of both forearms in 24 healthy volunteers. The measurements deducted from these scans were compared with the golden standard; direct circumference measurements with a measuring tape and volume measurements using water displacement volumetry. The interrater reliability and accuracy were calculated.
Results: The interrater reliability was 0.992 (p < 0.001) and 0.952 (p < 0.001) for circumference and volume measurements respectively. The dimensions obtained from with the 3D scanner were strongly correlated with their direct counterparts r = 0.977 (p < 0.001) and r = 0.893 (p < 0.001).
Conclusion: Based on the results from this study, the Structure Sensor 3D scanner has shown to be a reliable method for reproducible data on the forearm dimensions. Further research is necessary to investigate the use of these 3D scans in the process of creating 3D printed patient-specific orthoses in the treatment of DRF.
Introduction
The distal radius fracture (DRF) is one of the most common type of fracture, accounting for around 25% of fractures and up to 18% of all fractures in the elderly1. Non-displaced or stable fractures are commonly treated conservatively, which consists of a mineral splint to allow for trauma-related swelling. After one or two weeks, the swelling has reduced and the splint is replaced by a circular cast made of plaster which provides better support2,3. These traditional casts can be experienced as uncomfortable due to poor ventilation, weight and improper fit. Complications may occur depending on the experience of the individual applying the cast, such as cutaneous diseases, tendinopathies and arthralgia, or mal-union4-6. Excessive pressure from the cast could even result in neuropraxia7. A solution for these problems can be a patient-specific cast with appropriate fit and a ventilated structure. The design of these orthoses can be created using 3D printing, an innovative technique which is rapidly evolving in health care. Some entrepreneurs8 and explorative studies9-11 are already developing orthoses produced with 3D printing. These 3D printed orthoses could result in a uniform quality for every patient, with expected lower complication rates. However, in order to design 3D-printed casts, reliable measurements of the patient’s arm are necessary. A quick, easy and non-invasive method to obtain these data is a stereophotogrammetry12-14 and infra-red imaging15,16 based 3D scan. The arm of the patient will be scanned using this technology without radiation exposure. The scan will result in a 3D model of the arm, which is used to design 3D printed orthoses10,11. However, it is important to validate such techniques before clinical implementation. Therefore the aim of this study is to validate the use of a non-invasive portable 3D-scanner in obtaining forearm dimensions, by comparing the dimensions obtained from the 3D scan with forearm circumference using tape measurements, and volume using water displacement volumetry.
Materials and Methods
Procedure
The study was approved by the local attainability committee. The participants were employees or students of our institution. Informed consent was obtained from the participant before inclusion in this study.
All participants remained indoors in a room temperature-controlled environment. They did not engage any strenuous activity between or before measurements. The measurements were performed consecutively by three different observers. The direct circumference measurements were measured with a measuring tape and consisted of four circumferences along the forearm (Figure 1a):
1) 2 cm below the third metacarpal tuberosity
2) the wrist at ulnar styloid process
3) 10 cm proximal of the ulnar styloid process
4) 20 cm proximal of the ulnar styloid process
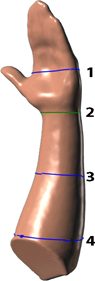
Figures 1a: Measurement points for direct circumferences of the forearm
The fourth measurement point was marked on the participant’s arm, the participant was instructed to slowly immerse their forearm with the wrist in a neutral position (Figure 1b) until that mark into a basin filled with water at room temperature. This position was held in place until the water drops from the overflow spout were more than 1 second apart estimated visually. Water expelled from the vessel was caught in a plain plastic measuring jug, the weight was determined as quickly as possible (to the nearest 0.1 gram) using electronic balance scales (Model i2000, Gason). The weight of the dry measuring jug was subtracted from the combined weight of the jug with the water and the result was recorded as the volume of displaced water.

Figures 1b: Immersion of the forearm in water for direct volume measurement of the forearm
3D scans of the forearm were made using a hand-held scanning device; the Structure Sensor (Occipital Inc., San Francisco, CA, USA), which is an iPad accessory based on infra-red structured light (Structure Sensor, 2016). The manufacturer-given scan accuracy of the Structure Sensor is 4 mm and the resolution 0.5 mm17. The participants were instructed to hold their arm stretched out in front of them, with fingers closed and thumb out, with the wrist in neutral position. Their other arm could be used for support with the hand placed proximal of the elbow to leave enough room for the scanner to fully scan every surface. The scan was made using the 3DSizeME app (TechMed3D, Quebec, Canada) in combination with the Structure Sensor. The data of the measurements were extracted using the MSoft 3.0 software (TechMed3D, Quebec, Canada).
Statistical analysis
The reliability of the Structure Sensor is defined by the interrater reliability in the digital measurements measured with the intra-class correlation coefficient (ICC). Pearson r correlations between the average direct and the digital measurements were calculated. The accuracy of the Structure Sensor is determined by the difference between the 3D-scanner and the average direct circumference measurements and average water displacement volumetry . Accuracy was evaluated with Bland - Altman plots18 as well as by calculating bias. Statistical tests were performed with IBM SPSS Statistics 25 and considered significant if p < 0.05.
Results
In total 24 healthy adults were included; 4 men and 20 women, aged 21-59 years (mean 34 ± 13 years).
The average measurements derived from the 3D scanner and the average direct measurements with standard deviation per observer are shown in Tables 1 and 2. The intra-class correlation coefficient (ICC) for all measurements with three different observers for the direct and scanner circumferences were 0.993 (p < 0.001) and 0.992 (p < 0.001), for the direct and scanner volumes the ICC were 0.997 (p < 0.001) and 0.952 (p < 0.001) respectively. Figures 2 and 3 display Pearson r correlations plots of the direct and digital circumference and volumetric measurements. Correlations of the direct measurements to their 3D scanner counterparts yielded Pearson r correlations of 0.977 (p < 0.001) for the circumference and 0.893 (p < 0.001) for the volumetric measurements. The maximum error for the circumference measurements was 17% and 19% for the volume measurements. The circumference measurements were on average larger than the direct measurements on all measure points.
Table 1: Dimensions derived from 3D scans
|
|
Measurement point 1 (mm) |
Measurement point 2 (mm) |
Measurement point 3 (mm) |
Measurement point 4 (mm) |
Volume (ml) |
|
Rater 1 |
193 ± 11 |
156 ± 10 |
205 ± 18 |
258 ± 23 |
998 ± 163 |
|
Rater 2 |
192 ± 11 |
156 ± 10 |
205 ± 18 |
258 ± 23 |
974 ± 158 |
|
Rater 3 |
194 ± 12 |
160 ± 10 |
211 ± 16 |
261 ± 18 |
1031 ± 143 |
Table 2: Direct measurements
|
|
Measurement point 1 (mm) |
Measurement point 2 (mm) |
Measurement point 3 (mm) |
Measurement point 4 (mm) |
Volume (ml) |
|
Rater 1 |
193 ± 12 |
156 ± 10 |
201 ± 18 |
246 ± 23 |
996 ± 173 |
|
Rater 2 |
193 ± 11 |
156 ± 9 |
202 ± 17 |
244 ± 24 |
991 ± 164 |
|
Rater 3 |
190 ± 10 |
155 ± 9 |
203 ± 18 |
246 ± 19 |
1005 ± 172 |
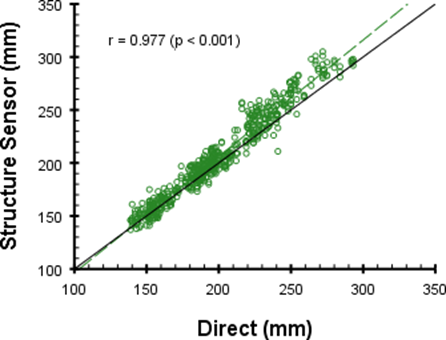
Figures 2: Pearson r correlation plot of direct and digital circumference measurements
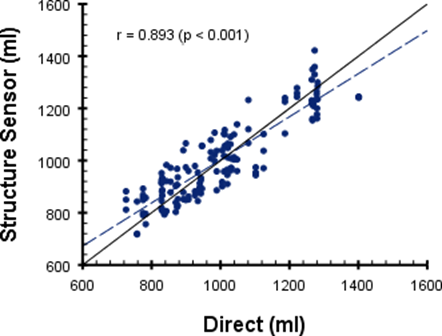
Figures 3: Pearson r correlation plot of direct and digital volumetric measurements
Discussion
This study evaluated the use of a portable and non-invasive 3D-scanner to obtain forearm dimensions, by comparing circumferences of the forearm and volume-based measurements to data obtained from the 3D scan. Measurements obtained by 3D scans are highly correlated with circumference as measured with a measuring tape. Volume measurements using the 3D scanner show a very good inter-rater reliability. Circumference measurements using the 3D-scanner show excellent inter-rater reliability with a difference in ICC of 0.001, which suggests that the scanner performs independently of the skill of the operator. This makes it a useful tool in everyday practice, as the scanner does not require special training of the staff.
The measured circumference in the 3D scans tends to be higher than the direct measurements (Fig. 4). This is consistent with other literature evaluating the Structure Sensor15,16. Volume measurements are averaged around zero (Fig. 5), suggesting that other dimensions are underestimated to compensate for the overestimated measure point circumferences. The discrepancy can be explained by 3D scanning the fingers. As these parts are difficult to scan. However, this study is technically not enforceable without measuring the complete forearm and hand. In the end, the fingers are not relevant for the final 3D-printed orthoses in DRF, since the orthoses stop just proximal of the metacarpophalangeal joints.
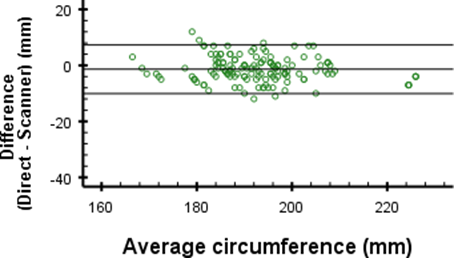
Figures 4a: Differences in direct and digital circumference measurements for measurement point 1
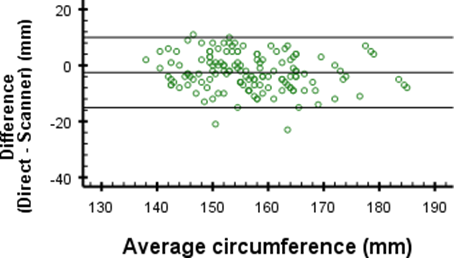
Figures 4b: Differences in direct and digital circumference measurements for measurement point 2
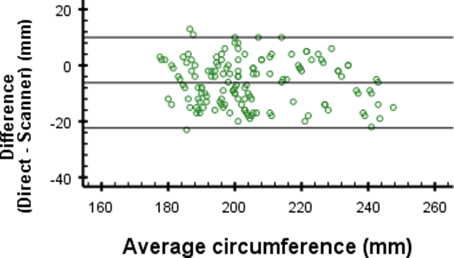
Figures 4c: Differences in direct and digital circumference measurements for measurement point 3
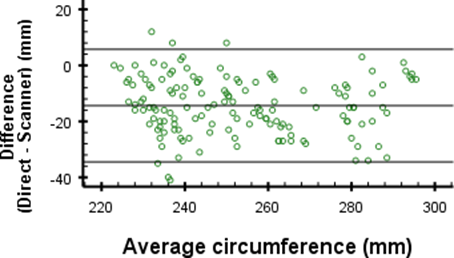
Figures 4d: Differences in direct and digital circumference measurements for measurement point 4
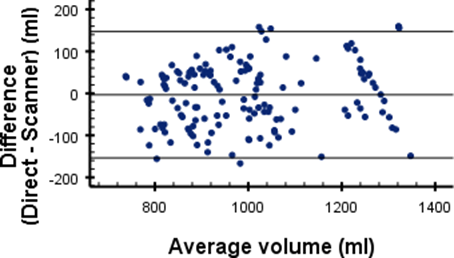
Figures 5: Differences in direct and digital volume measurements
A remarkable finding is the mean difference of -14.4 mm at measurement point 4 of the circumferences. This difference is much larger than -1.4 mm, -2.5 mm and -6.2 mm difference as found for the other measurement points (Figure 4). In Tables 1 and 2 this difference is seen between the mean circumferences from the 3D scan and direct measurements at point 4. Since the standard deviations are similar, this indicates that there may be a problem which causes an offset inaccuracy without influencing the precision. The cause of this error is most likely the angle at which the elbow was bent during measurement. When the elbow is flexed, the shape of the proximal part of the forearm changes due to muscle tension. During measurements, the angle of the elbow was not properly regulated, which could very well explain the large differences. When ignoring measurement point 4, the differences are close to the 4 mm accuracy given by the manufacturer17.
Clinical relevance
Capabilities of 3D-printed orthoses are widely evaluated in literature9-11,19,20. These orthoses can eliminate the problems with the traditional circular casts, such as improper fit and poor ventilation. It is not desirable to subject a patient with a DRF to extensive measurements with a measuring tape. Another way to obtain measurements from the patient’s arm is through CT or MRI, however in case of CT the patient is exposed to radiation21,22 and both cases are expensive and time-consuming23. The use of a portable and non-invasive 3D-scanner is a perfect way to gain the correct data. 3D scans can quickly and easily be made during regular outpatient visits thus improving patient convenience24. This study showed that the Structure Sensor is a reliable, easy to use and non-invasive method to obtain forearm measurements. We do not anticipate problems from the slightly overestimated circumference measurements, since some padding of the 3D printed orthoses is required. Because we proved that the overestimation is consistent in all measurements, a standard correction of the 4 mm overestimation can be made in the final model of the 3D print model. However, further research is required to investigate the actual differences when 3D printing the orthoses.
Limitations
An important note is that this study only includes healthy adults. Patient compliance is required to minimize motion artifacts, this could be challenging for patients with a DRF and especially in pediatric patients. However, we do not anticipate large problems, since the position of the arm and wrist for making a 3D scan is similar to the position required to apply the cast.
In this study the acquisition and measurement software (respectively 3DSizeME and MSoft) of TechMed3D was used for all scans and measurements. Other literature on the Structure Sensor using different acquisition and measurement software show similar results15,16, therefore we do not expect that usage of different software might result in different findings.
Conclusion
Obtaining forearm dimensions using the Structure Sensor 3D-scanner provides reproducible data of the circumference and volume. Results show a strong correlation between direct and scan measurements, and a high inter-rater reliability. This means that the scanner measures consistently independent of the operator. Further study is required to evaluate the fit of the orthoses after 3D printing.
Acknowledgements
The authors would like to thank Chris Aarts and Marije van der Vee for their participation in acquiring the measurements.
Author contributions
TdB and EH were involved in study design, interpretation of data and drafting the manuscript. HN was involved in data acquisition, interpretation of the data and drafting the manuscript. LvS and APtG were involved in data interpretation and critical revision of the manuscript.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Nellans KW, Kowalski E, Chung KC, et al. “The Epidemiology of Distal Radius Fractures,”. Hand Clin. 2012; 28(2): 113–125.
- American Academy of Orthopaedic Surgeons. Distal Radius Fractures [Internet]. Rosemont Illinois: American Academy of Orthopaedic Surgeons; 2013 [cited 2017 Oct 19]. Available from: http://orthoinfo.aaos.org/topic.cfm?topic=a00412(Level of Evidence = 5)
- Slutsky DJ. “Distal Radius Fractures,”. Green’s Operative Hand Surgery. 2005; 21(1783): 2005–2005.
- Tan V, Bratchenko W, Nourbakhsh A, et al. “Comparative analysis of intramedullary nail fixation versus casting for treatment of distal radius fractures.” The Journal of hand surgery. 2012; 37(3): 460–468.
- Kamath AF, Zurakowski D, Day CS. “Low-Profile Dorsal Plating for Dorsally Angulated Distal Radius Fractures: An Outcomes Study,”. The Journal of Hand Surgery. 9 2006; 31(7): 1061–1067.
- Inglis M, McClelland B, Sutherland lM, et al. “Synthetic versus plaster of Paris casts in the treatment of fractures of the forearm in children,”. The Bone & Joint Journal. 9 2013; 95-B(9): 1285–1289.
- Walker RW, Draper E, Cable J. “Evaluation of pressure beneath a split above elbow plaster cast,”. Annals of the Royal College of Surgeons of England. 2000; 82(5): 307–310.
- ActiveArmor, “https://activarmor.com/,” 2018.[Online] Available: https://activarmor.com/
- Faria A, Couto M, Santos JD, et al. “Additive Manufacturing of Custom-Fit Orthoses for the Upper Limb,”. 7o Congresso Nacional De Biomecanica. March, 2017.
- Lin H, Shi L, Wang D, “A rapid and intelligent designing technique for patient-specific and 3D-printed orthopedic cast,”. 3D Printing in Medicine. 12 2015; 2(1): 4.
- Chen YJ, Lin H, Zhang X, et al. “Application of 3Dprinted and patient-specific cast for the treatment of distal radius fractures: initial experience,”. 3D Printing in Medicine. 2017; 3(1): 11.
- Verhulst AC, Wesselius TS, Glas HH, et al. “Accuracy and reproducibility of a newly developed tool for volume measurements of the arm using 3D stereophotogrammetry,”. Journal of Plastic Reconstructive and Aesthetic Surgery. 2017; 70(12): 1753–1759.
- Seminati E, Talamas DC, Young M, et al. “Validity and reliability of a novel 3D scanner for assessment of the shape and volume of amputees residual limb models,”. PLoS ONE. 2017; 12(9): 1–16.
- Kofman R, Beekman AM, Emmelot CH, et al. “Measurement properties and usability of non-contact scanners for measuring transtibial residual limb volume,”. Prosthetics and Orthotics International. 2017; 1: 1–8.
- Beaumont CA, Knoops PG, Borghi A, et al. “Three-dimensional surface scanners compared with standard anthropometric measurements for head shape,”. Journal of CranioMaxillofacial Surgery. 6 2017; 45(6): 921–927.
- Knoops PG, Beaumont CA, Borghi A, et al. “Comparison of three-dimensional scanner systems for craniomaxillofacial imaging,”. Journal of Plastic Reconstructive and Aesthetic Surgery. 2017; 70(4): 441–449.
- Occipital Inc. “Structure Sensor,” 2018. [Online]. Available: https://structure.io/
- Bland JM, Altman DG. “Statistical methods for assessing agreement between two methods of clinical measurement,”. Lancet. 1986; i: 307–310.
- Li J, Tanaka H. “Feasibility study applying a parametric model as the design generator for 3Dprinted orthosis for fracture immobilization,”. 3D Printing in Medicine. 2018; 4(1): 1.
- Baronio G, Harran S, Signoroni A. “A Critical Analysis of a Hand Orthosis Reverse Engineering and 3D Printing Process.” Applied bionics and biomechanics. 2016; 2016: 8347478.
- Fazel R, Krumholz HM, Wang Y, et al. “Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures,”. New England Journal of Medicine. 8 2009; 361(9): 849–857.
- Smith-Bindman R. “Is Computed Tomography Safe?”. New England Journal of Medicine. 7 2010; 363(1): 1–4.
- Hillman BJ, Goldsmith JC. “The Uncritical Use of High-Tech Medical Imaging,”. New England Journal of Medicine. 7 2010; 363(1): 4–6.
- Schneider AJ, Zonderland IME, Bots ICAJ. “Capacity planning for waiting list management at the Radiology department of Leiden University Medical Center,”. 2011.