Redo Pulmonary and Tricuspid Valve Replacement using St. Jude Medical Epic Bioprostheses in a Patient with Repaired Tetralogy of Fallot with Severe Pulmonary and Tricuspid Valvular Regurgitation: A Video Presentation
Ujjwal K. Chowdhury*, Sukhjeet Singh, Niwin George, Lakshmi Kumari Sankhyan, Sanjoy Sengupta, Sushamagayatri B, Parag Gharde, Vishwas Malik, Sreenita Chowdhury
Cardiothoracic Sciences Centre, All India Institute of Medical Sciences, India
Introduction
The wide spectrum of right ventricular outflow tract obstruction and pulmonary arterial anatomy encountered in tetralogy of Fallot necessitates an individualized surgical approach. The morphology of the pulmonary valve and the method of right ventricular outflow tract reconstruction are indeed the most important determinants of later pulmonary valve incompetence1-9.
The ideal right ventricular outflow tract reconstruction involves striking the dedicate balance, whereby the surgeon avoids both inadequate resection and excessive transannular patching. Decisions made at the time of intracardiac repair of tetralogy of Fallot have a definite impact on the performance of repair over the subsequent 20-30 years and beyond1-9.
Although, the primary decision-making on the requirement of transannular patch is indeed the size of the pulmonary valve ring, postoperative peak systolic right ventricle-to-left ventricle (Prv/Plv) can guide the surgeons in borderline cases. Many surgical centers would consider using a transannular patch if immediate post repair Prv/Plv is >0.7. Increased postoperative Prv/Plv is widely accepted as a risk factor for adverse postoperative outcomes as well as long-term mortality1-5. A postoperative Prv/Plv between 0.8 to 1.0 without TAP and >0.85 with TAP adversely affects survival1-7.
Despite the risk of reoperations, a large number of studies have documented satisfactory long-term survival rates in repaired tetralogy of Fallot, with a 30-year survival rate ranging from 86% to 91.7%4-12.
In general, the patient survival rate declines significantly 15 years after intracardiac repair with mortality rates increasing from 0.12% per year in the first 15 years to 0.39% per year after 15 years12. The reported freedom from reoperations after intracardiac repair of tetralogy of Fallot in the neonatal period at 1 month, 1 year and 5 years have been 100%, 93% and 63% respectively. The relatively high rate was influenced by the complex anatomy in symptomatic neonates with tetralogy of Fallot, a high incidence of non-confluent pulmonary arteries, branch pulmonary arteries and conduit insertion4-12.
The most common indications for reoperation following intracardiac repair of tetralogy of Fallot are the result of long-term complications related to right ventricular outflow tract such as severe pulmonary regurgitation, residual right ventricular outflow tract obstruction and conduit failure4-12.
Other reasons for reoperation include residual ventricular septal defect, pulmonary valvular stenosis, left pulmonary artery stenosis, aortic valvular stenosis or insufficiency. The indication for reoperation in residual right ventricular outflow tract is controversial. Generally, surgery is indicated for right ventricular outflow tract gradient is more than 60 mmHg4-12.
The effect of transannular patch on long-term survival and reoperation remains controversial. While authorities experienced no difference in long-term survival and reoperation, other investigators have implicated transannular patch as a risk factor for re-intervention and reduced long-term survival4-13.
Nevertheless, literature documents a lot of experimental work demonstrating the deleterious effects of chronic pulmonary regurgitation on right ventricular function, right ventricular volume and exercise performance4-13.
Several large series of pulmonary valve replacement for correction of chronic pulmonary regurgitation have been published12-14. However, the indications have been ill-defined in most of the series. Influences are further complicated by the poor correlation between right ventricular function and exercise12-14.
In most reported series, the indications for pulmonary valve replacement have been primarily related to the appearance of symptoms of right ventricular failure. However, majority of the symptomatic patients will have irreversible right ventricular dysfunction and result in minimal benefits from pulmonary valve replacement15-17. It may be a reasonable to follow-up asymptomatic or minimally symptomatic patients with repaired tetralogy of Fallot to follow-up with serial echocardiography, serial exercise studies and radionuclide studies15-17. Although the cardiac magnetic resonance imaging is the best option, it may not widely available17-19.
Literature is divided on the timing of pulmonary valve replacement. Analysis of the published investigations categorize the indications of pulmonary valve replacement in repaired tetralogy of Fallot as under:
1. Symptomatic patients with long-standing severe pulmonary regurgitation and right ventricular dilation with or without right ventricular dysfunction20.
2. Clinical deterioration of functional status with serial deterioration of exercise capacity on treadmill testing20.
3. Asymptomatic patients with severe pulmonary regurgitation and evidence of progressive right ventricle dilation and dysfunction on investigations. These include- a) serial prolongation of QRS duration on ECG; b) echocardiographic evidence of severe pulmonary regurgitation i.e. pulmonary regurgitation index (PRi) <0.7720; c) pulmonary pressure half time <100 msec15; right ventricular dilatation index; e) ratio of right ventricular end-diastolic dimension and left ventricular end-diastolic dimension (RVDi) >213; f) cardiac magnetic resonance evidence of severe pulmonary regurgitation i.e. pulmonary regurgitant fraction >40%21; g) significant right ventricular dilatation (right ventricular end-diastolic volume / body surface area) > 150 ml/m2 22 right ventricular systolic dysfunction (right ventricular ejection fraction <40%)20.
4. Patients with serious ventricular arrhythmias (sustained monomorphic ventricular tachycardia) associated with severe pulmonary regurgitation and dilated right ventricle20.
5. Asymptomatic or symptomatic patients with moderate to severe pulmonary regurgitation and concomitant hemodynamically significant associated lesions requiring surgical management.
6. Regarding tricuspid regurgitation, some investigators believe that it may be hazardous to delay pulmonary valve replacement until after the onset of this additional lesion, since this may present irreversible right ventricular dysfunction13,20.
Mechanical prostheses, heterografts, autologous pericardial valves, bovine jugular valves mounted on stents have all been used for pulmonary valve replacement. The use of a mechanical valve in the pulmonary position has fallen out of favour due to high incidence of thromboembolic phenomena and valve failure21-23.
Homograft valves have been widely used for pulmonary valve replacement with 20% year freedom from reoperation in 40% of patients24. Some authors report the durability of cryopreserved pulmonary homografts similar to pulmonary homografts used for the Ross procedure25.
Severe pulmonary regurgitation in repaired tetralogy of Fallot is sometimes associated with aneurysmal right ventricular outflow tract patches. When the use of homografts is contemplated, it is important to restore the geometry of the ventriculoarterial junction to prevent distortion of the homograft. A stented bioprosthetic may be a preferred option.
The Contegra, a new biological valved conduit consisting of a glutaraldehyde-preserved homologous bovine jugular vein with a trileaflet venous valve has also reported to have good results at 2 years follow-up26.
The requirement and clinical implications of pacemaker implantation after tricuspid valve operation is less well documented. A limited number of studies have shown that the need of pacemaker implantation after a tricuspid valve operation is higher, at 13% to 28% than after other valve interventions27,28. The use of an annuloplasty ring rather than a Devega annuloplasty or prosthetic tricuspid valve is an independent predictor of pacemaker requirement28.
Insertion of an endocardial pacemaker electrode in patients with tricuspid valve replacement may lead to a high percentage of secondary tricuspid regurgitation29. Application of epicardial lead systems is an alternative system but is associated with high packing thresholds30. Data on His bundle pacing via access through the coronary sinus in the presence of an artificial tricuspid valve are limited and mainly retrieved from case reports29,31. In a retrospective study, Noheira and associates described so far the largest group of 23 patients in whom cardiac pacing via the coronary sinus was applied comparing it with traditional pacing of the right ventricle32.
We report here-in a 25-year-old female patient of repaired tetralogy of Fallot with severe tricuspid and pulmonary regurgitation undergoing tricuspid and pulmonary valve replacement using St. Jude Epic bioprosthesis under moderately hypothermic cardiopulmonary bypass and St. Thomas based cold blood cardioplegia. She underwent intracardiac repair of tetralogy of Fallot of 2 years of age. The importance of elective institution of femoro-femoral bypass prior to sternotomy and cardioplegic arrest during tricuspid valve replacement in cases of associated persistent foramen ovale has been highlighted.
Surgical Techniques
Following systemic heparinisation, elective right femoral arteriovenous cannulation was done using long femoral arterial and venous cannulae (Edwards Lifesciences LLC, One Edwards Way, Irvine, CA, USA).
Under cardiopulmonary bypass, secondary median sternotomy was performed with the heart decompressed on bypass. The pericardium overlying the aorta, right ventricular outflow tract and superior vena cava was dissected.
The superior caval vein was dissected and cannulated directly using an angled metal tipped venous cannula and drained directly into the oxygenator. The intrapericardial inferior caval vein was dissected and looped for later occlusion.
The ascending aorta was dissected free from the main pulmonary artery and right pulmonary artery for later selective aortic cross-clamp. The aorta was cross-clamped using an atraumatic aortic vascular clamp. Myocardial protection was achieved by intermittent administration of St. Thomas (II) based cold blood cardioplegia (4: 1) and ice cold saline.
After clamping the inferior caval vein, the right atrium was directly incised in between stay sutures 1.5 cm parallel and posterior to the atrioventricular groove. A 14 Fr sump suction vent was inserted through the patent foramen ovale for left heart decompression. The main pulmonary artery was opened vertically in between stay sutures.
The tricuspid valve was checked for competence using normal saline and was deemed irreparable. The tricuspid valve was replaced using a 29 mm St. Jude Epic bioprosthesis (St. Jude Medical; St. Paul, MN, USA) sutured with interrupted pledgeted mattress suture of 2-0 Ticron sutures. The patent foramen ovale was closed directly using 4-0 polypropylene suture. The right atrium was closed in two layers using polypropylene suture. The cardiac chambers were covered using a patch of bovine pericardium.
The aortic cross clamp was released, thus restoring myocardium perfusion. At 29 mm St. Jude Epic bioprosthesis (St. Jude Medical; St. Paul, MN, USA) was implanted in the orthotopic location using interrupted pledgeted mattress suture of 2-0 Ticron (M/s Covidien Domingo, Dominican Republic, USA) sutures. The main pulmonary artery was closed directly using 4-0 polypropylene sutures (Johnson and Johnson Ltd., Ethicon, LLC, San Lorenzo, USA) in two layers.
Results
The patient was weaned off cardiopulmonary bypass on dopamine 7.5 µg/kg/min, dobutamine 7.5 µg/kg/min, adrenaline 0.1 µg/kg/min and elective intra-aortic balloon support for low cardiac output. She was extubated after 24 hours and was weaned-off IABP support after 96 hours. At 10 months follow-up she is in New York Heart Association functional class I with left ventricular ejection fraction 0.60 in normal sinus rhythm. Postoperative echocardiography demonstrated normal bioprosthetic tricuspid and pulmonary valvular function without paravalvular leak (Figures 1A-1D).
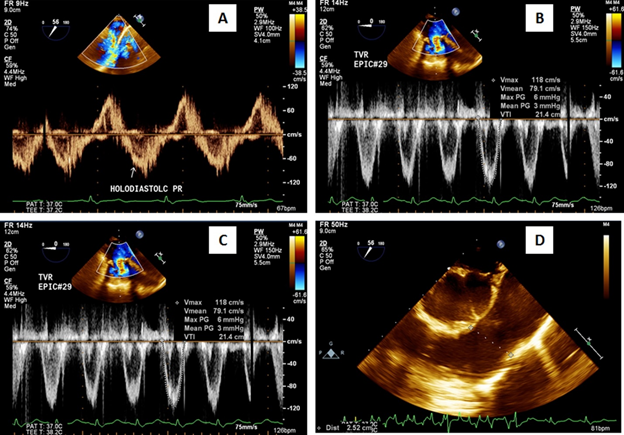
Figure 1A-1D: 1A- Preoperative Doppler echocardiography showing holodiastolic pulmonary regurgitation and 1B-1D- postoperative echocardiography showing normally functioning tricuspid and pulmonary valves without valvular regurgitation or paravalvular leak.
Conclusions
Elective institution of cardiopulmonary bypass through femoro-femoral arterio-venous cannulation prior to sternotomy prevents accidental injury to cardiac chambers and great vessels during sternal entry in patients with repaired tetralogy of Fallot, with dilated right ventricle secondary to severe pulmonary and tricuspid regurgitation with previously excised pericardium. Although tricuspid and pulmonary valve replacement can be performed in a beating perfused heart, cardioplegic arrest is desirable in patients with a persistent foramen ovale to prevent accidental air embolism.
Pulmonary valve replacement should be performed in symptomatic patients with progressive right ventricular dilatation and/or dysfunction before irreversible right ventricular dysfunction ensues.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of the article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
References
- Hickey EJ, Veldtman G, Bradley TJ, et al. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg. 2009; 35: 156 -164; discussion 164.
- Chowdhury UK, Pradeep KK, Patel CD, et al. Noninvasive assessment of repaired tetralogy of Fallot by magnetic resonance imaging and dynamic radionuclide studies. Ann Thorac Surg. 2006; 81(4): 1436-1442.
- Nollert G, Fischlein T, Bohmer C, et al. Long term survival in patients with repair of tetralogy of Fallot: 36 year follow up of 490 survivors of the first year after surgical repair. J Am Coll Card. 1997; 30: 1374-1383.
- Katz NM, Blackstone EH, Kirklin JW, et al. Late survival and symptoms after repair of tetralogy of Fallot. Circualtion. 1982; 65: 403-410.
- Chowdhury UK, Pradeep KK, Patel CD, et al. Non-invasive assessment of repaired tetralogy of Fallot by magnetic resonance imaging and dynamic radionuclide studies. Ann Thorac Surg, 2006; 81: 1436-42.
- Chowdhury UK, Jha A, Hasija S, et al. Relationship of tissue Doppler-derived myocardial velocities to peak systolic right-to-left ventricular pressure ratio in repaired tetralogy of Fallot. Journal of Archives of Pediatrics, 2018; Issue 2: JPED 156.
- Chowdhury UK, Jha A, Ray R, et al. Histopathology of the right ventricular outflow Tract and its relation to hemodynamics in patients with repaired tetralogy of Fallot. Journal of Thoracic and Cardiovascular Surgery. 2019: 1-11. Available online (in press) doi: https://doi.org/10.1016/j.jtcvs.2019.05.013.
- Gatzoulis MA, Clark AL, Cullen S, et al. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot. Restrictive physiology predicts superior exercise performance. Circulation. 1995; 91: 1775-1781.
- Nollert G, Fischlein T, Bouterwek S, et al. Long-term results of total repair of tetralogy of Fallot in adulthood: 35 years follow-up in 104 patients corrected at the age of 18 or older. Thorac Cardiovasc Surg. 1997; 45: 178-81.
- Nakazawa M, Shinohara T, Sasaki A, et al. Arrhythmias late after repair of tetralogy of Fallot: a Japanese multicentre study. Circ J. 2004; 68: 126 -130.
- Knott-Craig CJ, Elkins RC, Lane MM, et al. A 26-year experience with surgical management of tetralogy of Fallot: risk analysis for mortality or late reintervention. Ann Thorac Surg. 1998; 66: 506-11.
- Oechslin EN, Harrison DA, Harris L, et al. Reoperation in adults with repair of tetralogy of Fallot: indications and outcomes. J Thorac Cardiovasc Surg. 1999; 118: 245-51.
- Misbach GA, Turley K, Ebert PA. Pulmonary valve replacement for regurgitation after repair of tetralogy of Fallot. Ann Thorac Surg. 1983; 36: 684–91.
- Cheung EW, Wong WH, Cheung YF. Meta-analysis of pulmonary valve replacement after operative repair of Tetralogy of Fallot. Am J Cardiol. 2010; 106: 552-7.
- Silversides CK, Veldtman GR, Crossin J, et al. Pressure half-time predicts hemodynamically significant pulmonary regurgitation in adult patients with repaired tetralogy of fallot. J Am Soc Echocardiogr. 2003; 16: 1057-62.
- Conte S, Jashari R, Eyskens B, et al. Homograft valve insertion for pulmonary regurgitation late after valveless repair of right ventricular outflow tract obstruction. Eur J Cardiothorac Surg. 1999; 15: 143– 9.
- Therrien J, Siu SC, McLaughlin PR, et al. Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J Am Coll Cardiol. 2000; 36: 1670– 5.
- Hazekamp MG, Kurvers MM, Schoof PH, et al. Pulmonary valve insertion late after repair of Fallot’s tetralogy. Eur J Cardiothorac Surg. 2001; 19: 667– 70.
- Vliegen HW, van Straten A, de Roos A, et al. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of Fallot. Circulation. 2002; 106: 1703 – 7.
- Geva T. Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg. 2006; 11-22.
- Graham Jr TP. Management of pulmonary regurgitation after tetralogy of Fallot repair. Curr Cardiol Rep. 2002; 4: 63– 7.
- Finck SJ, Puga FJ, Danielson GK. Pulmonary valve insertion during reoperation for tetralogy of Fallot. Ann Thorac Surg. 1988; 45: 610– 3.
- Schlichter AJ, Kreutzer C, Mayorquim RC, et al. Long-term follow-up of autologous pericardial valved conduits. Ann Thorac Surg. 1996; 62: 155–60.
- Caldarone CA, McCrindle BW, Van Arsdell GS, et al. Independent factors associated with longevity of prosthetic pulmonary valves and valved conduits. J Thorac Cardiovasc Surg. 2000; 120: 1022 – 30 [discussion 1031].
- Oury JH, Hiro SP, Maxwell JM, et al. The Ross procedure: current registry results. Ann Thorac Surg. 1998; 66: S162 – 5.
- Breymann T, Thies WR, Boethig D, et al. Bovine valved venous xenografts for RVOT reconstruction: results after 71 implantations. Eur J Cardiothorac Surg. 2002; 21: 703– 10 [discussion 710].
- Scully HE, Armstrong CS. Tricuspid valve replacement. Fifteen years of experience with mechanical prostheses and bioprostheses. J Thorac Cardiovasc Surg. 1995; 109: 1035– 41.
- Jokinen JJ, Turpeinen AK, Pitkanen O, et al. Pacemaker therapy after tricuspid valve operations: implications on mortality, morbidity, and quality of life. Ann Thorac Surg. 2009; 87: 1806-14.
- Eleid MF, Blauwet LA, Cha YM, et al. Bioprosthetic tricuspid valve regurgitation associated with pacemaker or defibrillator lead implantation. J Am Coll Cardiol. 2012; 59: 813- 818.
- Rydlewska A, Boczar K, Lelakowski J. Coronary sinus pacing in patients after tricuspid valve surgery with complete atrioventricular block. Cardiol Cardiovasc Med. 2019; 3 (5): 369-372.
- Mazine A, Bouchard D, Moss E, et al. Transvalvular pacemaker leads increase the recurrence of regurgitation after tricuspid valve repair. Ann Thorac Surg. 2013; 96: 816-822.
- Noheria A, vanZyt M, Scott LR, et al. Singlesite ventricular pacing via the coronary sinus in patients with tricuspid valve disease. Europace. 2018; 20: 636-642.